
Digital Endpoints for Clinical Trials
Real-time monitoring of medication efficacy and side effects.

Features Grid
Digital endpoints and real-time monitoring for pharmaceutical clinical trials.
Digital Endpoints
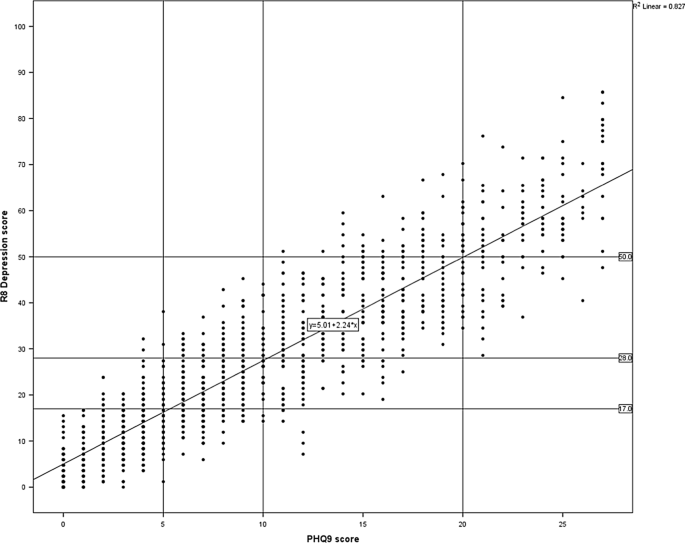
Validated measures of depression & anxiety.
Rapid Non-Response Detection
Within 4–6 days.
Post-Marketing Surveillance
Real-world evidence generation.
Evidence Section
Olanzapine response prediction study and Lithium outcomes audits demonstrate clinical effectiveness.
Medication Effectiveness Tracking
Benefits
Reduced Trial Costs
Faster recruitment and early efficacy detection reduce overall costs.
Higher-Quality Regulatory Submissions
Digital endpoints provide robust data for regulatory approval.
Continuous Monitoring Beyond Phase IV
Post-marketing surveillance and real-world evidence generation.
Accelerate Drug Development
Reduce trial timelines, improve data quality, and increase success rates with Psynary's digital clinical trial platform.
Integrate Psynary into your next trial
Partner with Psynary to accelerate drug development with digital endpoints and real-time patient monitoring.